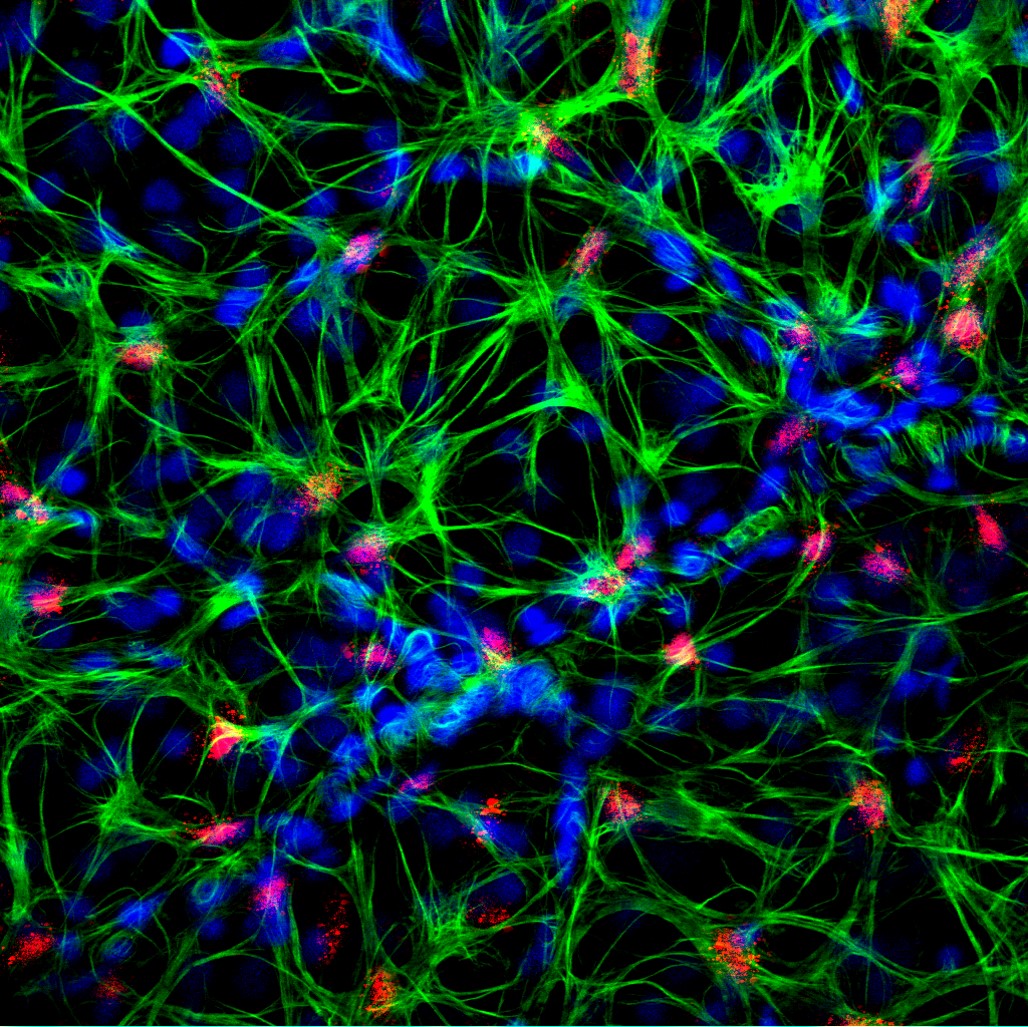
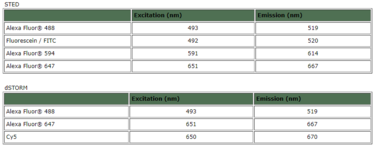
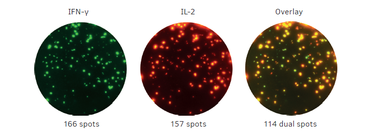
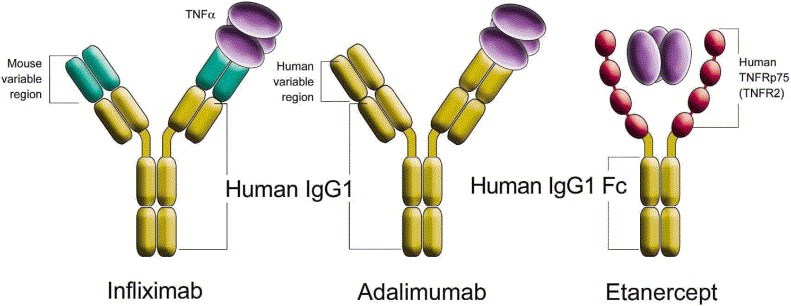
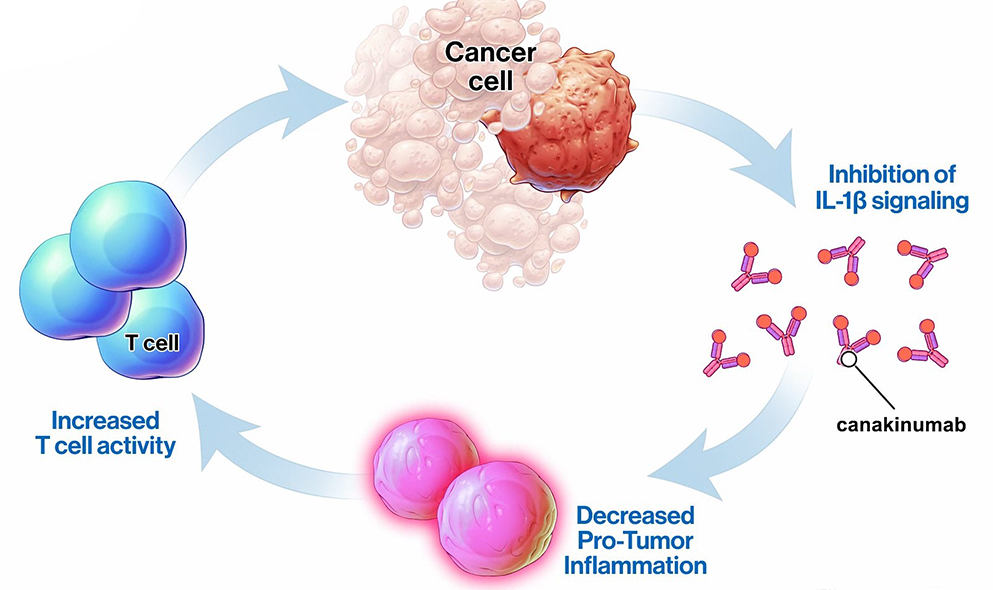
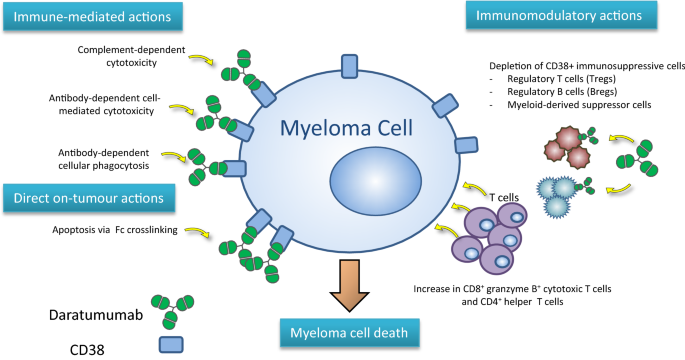
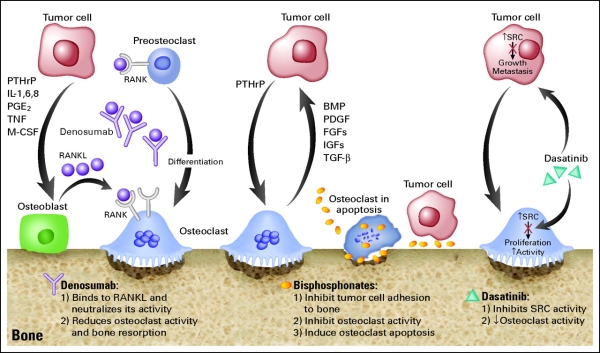
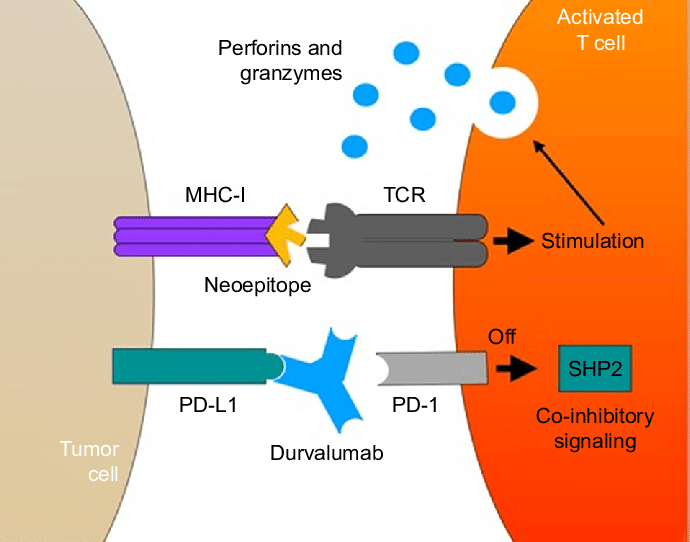
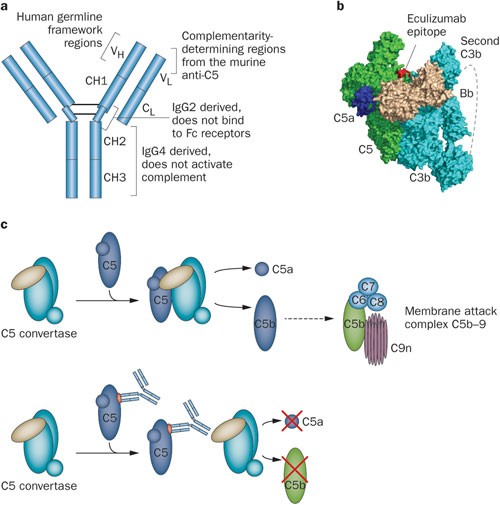
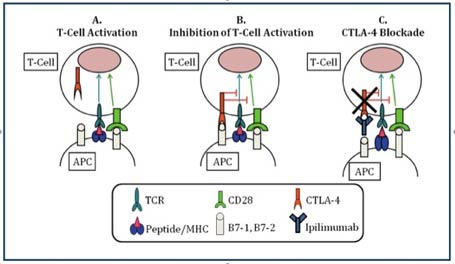
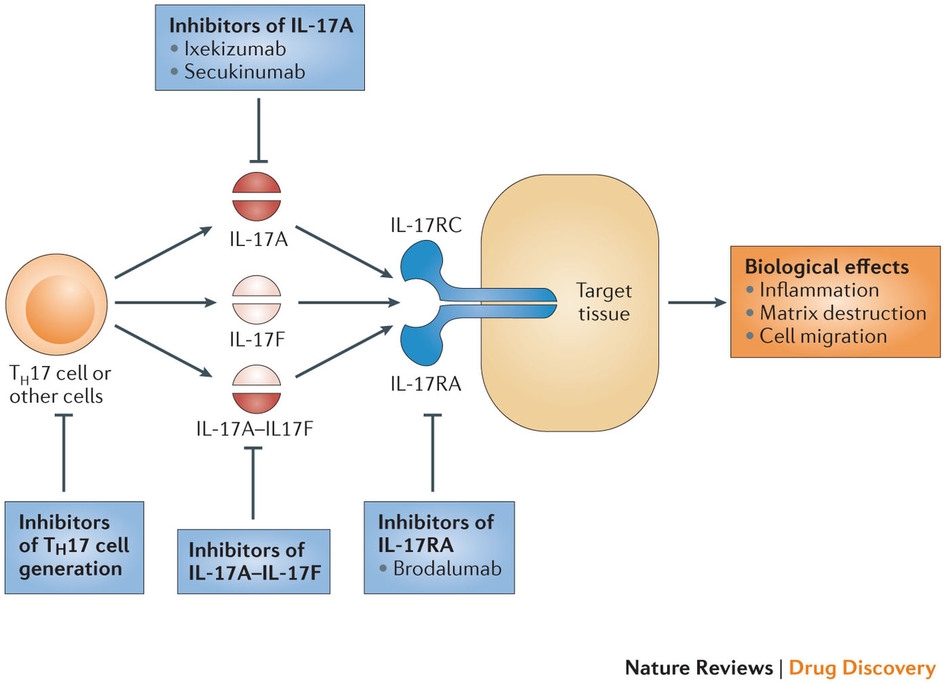
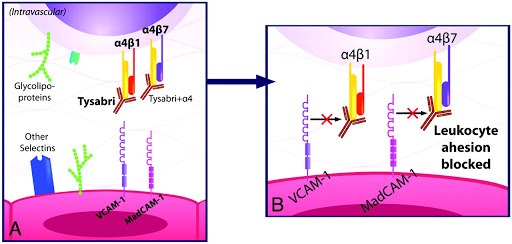
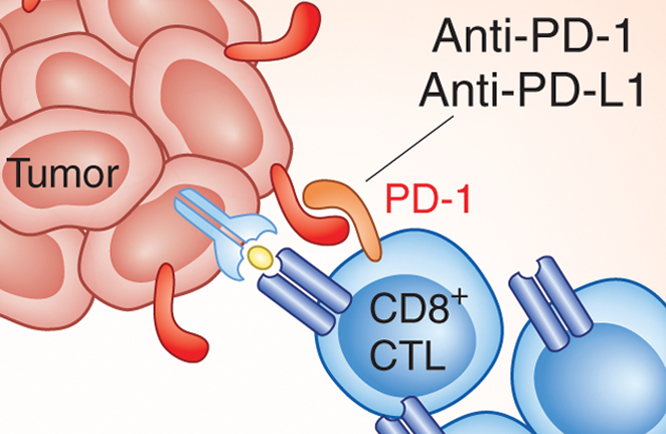
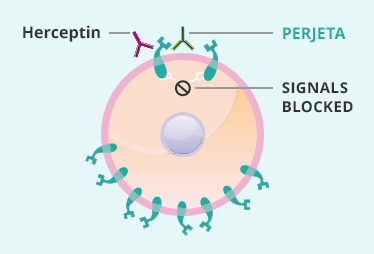
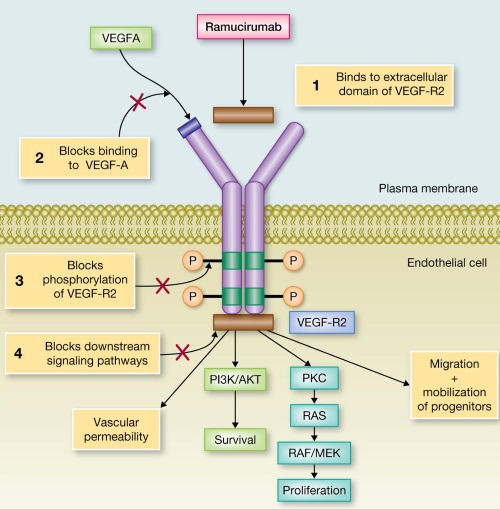
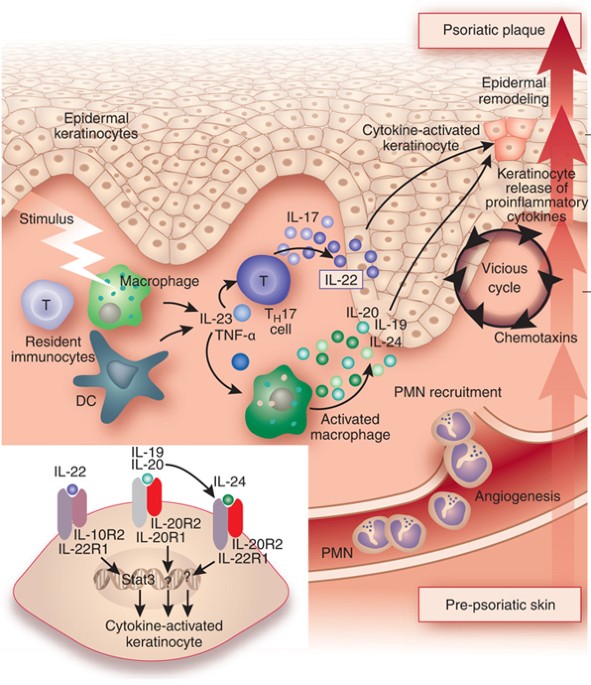
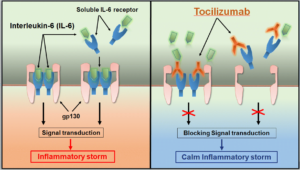
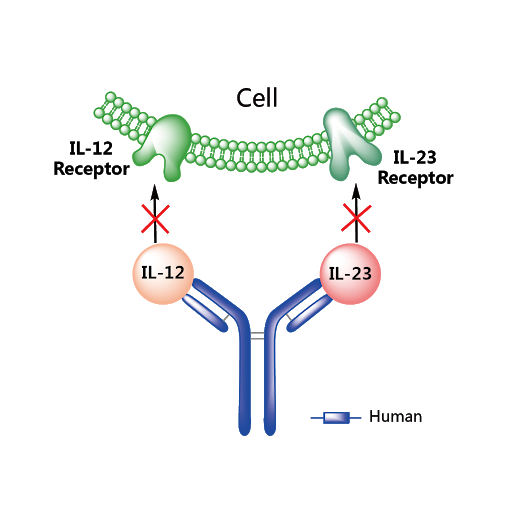
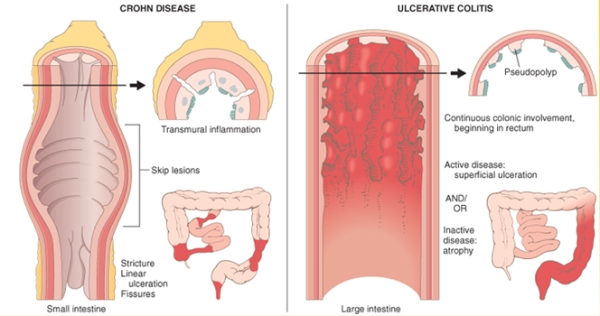
1 Lombardi S, Bernardoni C, Bertolucci D, et al. Biologic therapies in rheumatic diseases: drug and anti-drug antibody levels and clinical efficacy. Journal of Autoimmunity and Cell Responses, Volume 4, Article 1, 2017.**
2 Gregoritza M, Messmann V, Abstiens K, Brandl FP, Göpferich AM, Controlled antibody release from degradable thermoresponsive hydrogels cross-linked by Diels-Alder chemistry, Biomacromolecules, Just Accepted Manuscript, 22 Jun 2017.
3 Aldrich MB, Velasquez FC, Kwon S, Lymphatic delivery of etanercept via nanotopography improves response to collagen-induced arthritis, et al., Arthritis Research & Therapy 19:116, 2017.**
4 Kolar M, Duricova D, Bortlik M, et al., Infliximab Biosimilar (Remsima TM ) in Therapy of Inflammatory Bowel Diseases Patients: Experience from One Tertiary Inflammatory Bowel Diseases Centre, Digestive Disease, 35:91–100, 2017.
5 Gibellini L, De Biasi S, Bianchini E, et al. Anti-TNF-α Drugs Differently Affect the TNFα-sTNFR System and Monocyte Subsets in Patients with Psoriasis. Richard Y, ed. PLoS ONE. 11(12), 2016.**
6 Julsgaard M, Christensen LA, Gibson PR, Gearry RB, Fallingborg J, Hvas CL,Bibby BM, Uldbjerg N, Connell WR, Rosella O, Grosen A, Brown SJ, Kjeldsen J,Wildt S, Svenningsen L, Sparrow MP, Walsh A, Connor SJ, Radford-Smith G, LawranceIC, Andrews JM, Ellard K, Bell SJ. Concentrations of Adalimumab and Infliximab in Mothers and Newborns, and Effects on Infection. Gastroenterology. Jul;151(1):110-9, 2016.**
7 Jonathan GG, Beatriz AÁ, Nazco C GJ, Norberto BL, Fernando GN. Plasma levels of trastuzumab in gastric cancer: Case report. J Oncol Pharm Pract. Sep 23, 2016.**
8 Choi SY, Kang B, Lee JH, Choe YH. Clinical Use of Measuring Trough Levels and Antibodies against Infliximab in Patients with Pediatric Inflammatory Bowel Disease. Gut Liver. Sep 9 2016.**
9 Chen L., et al., Efficient Production of a Bioactive Bevacizumab Monoclonal Antibody Using the 2A Self-cleavage Peptide in Transgenic Rice Callus. Frontiers in Plant Science August 2016 | Volume 7 | Article 1156.**
10 Farkas K., et al., Efficacy of infliximab biosimilar CT-P13 induction therapy on mucosal healing in ulcerative colitis. Journal of Crohn’s and Colitis Advance Access published April 21, 2016.
11 Gutiérrez A, Zapater P, Juanola O, Sempere L, García M, Laveda R, Martínez A, Scharl M, González-Navajas JM, Such J, Wiest R, Rogler G, Francés R. Gut Bacterial DNA Translocation is an Independent Risk Factor of Flare at Short Term in Patients with Crohn’s Disease. Am J Gastroenterol. Apr;111(4):529-40, 2016.
12 Won Jae Song, Ben Kang, So Yoon Choi, and Yon Ho Choe. Adalimumab Treatment in Pediatric-Onset Crohn’s Disease Patients after Infliximab Failure: A Single Center Study. Pediatr Gastroenterol Hepatol Nutr. Jun; 19(2): 116–122, 2016.**
13 Hayashi S, et al., Early Prognostic Factors Associated with the Efficacy of Infliximab Treatment for Patients with Rheumatoid Arthritis with Inadequate Response to Methotrexate. Rheumatol Ther (2016) 3:155–166 **
14 Bortlik M., et al., Discontinuation of anti-tumor necrosis factor therapy in inflammatory bowel disease patients: a prospective observation. Scandinavian Journal of Gastroenterology, 2016 vol. 51, no. 2, 196–202.
15 Al-Karkhi M.A., et al., Correlation between Anti-infliximab and Anti-CCP Antibodies Development in Patients with Rheumatoid Arthritis Treated with Infliximab in Baghdad Teaching Hospital. IOSR Journal of Dental and Medical Sciences, Volume 14, Issue 11 Ver. IV (Nov. 2015), PP 95-100.**
16 Kui R, Gál B, Gaál M, Kiss M, Kemény L1, Gyulai R. Presence of antidrug antibodies correlates inversely with the plasma tumor necrosis factor (TNF)-α level and the efficacy of TNF-inhibitor therapy in psoriasis. J Dermatol. Sep;43(9):1018-23, 2016.**
17 Bodini G., Edoardo G. Giannini, Edoardo V. Savarino, and Vincenzo Savarino. Adalimumab Trough Levels and Response to Biological Treatment in Patients With Infl ammatory Bowel Disease: A Useful Cutoff in Clinical Practice. Am J Gastroenterol. Vol. 110, March, 2015.
18 Juanola O., et al., Anti-TNF-alpha loss of response is associated with a decreased percentage of FoxP3+ T cells and a variant NOD2 genotype in patients with Crohn’s disease. J Gastroenterol (2015) 50:758–768.
19 Fujita T, Murata Y, Hemmi S, Kajiwara M, Yabuki M, et al. (2015) Persistent Complement Activation is Associated with Insulin Resistance and Chronic Inflammation in Overweight Patients with Type 2 Diabetes with Dyslipidemia. Int J Immunol Immunother 2:007, 2015. **
20 Malíckova K, et al., Serum trough infliximab levels: A comparison of three different immunoassays for the monitoring of CT-P13 (infliximab) treatment in patients with inflammatory bowel disease, Biologicals 2015.
21 Farkas K. et al, Efficacy of the new infliximab biosimilar CT-P13 induction therapy in Crohn’s disease and ulcerative colitis experiences from a single center. Expert Opin. Biol. Ther. 15:(9) 2015.
22 Lee Y.M. et al, Infliximab ‘‘Top-Down’’ Strategy is Superior to ‘‘Step-Up’’ in Maintaining Long-Term Remission in the Treatment of Pediatric Crohn Disease. JPGN 60: (737–743), 2015.
23 Al-Karkhi M.A., et al., Development of Antibodies against Infliximab in Iraqi Patients with Rheumatoid Arthritis. J Fac Med Baghdad, 57: (241-243), 2015.**
24 Bergen T.V., et al., Complementary effects of bevacizumab and MMC in the improvement of surgical outcome after glaucoma filtration surgery. Acta Ophthalmologica 2015, 667-678.**
25 Aydın C, AtaoÄŸlu H., Demonstration of β-1,2 Mannan Structures Expressed on the Cell Wall of Candida albicans Yeast Form But Not on the Hyphal Form by Using Monoclonal Antibodies Mikrobiyol Bul 49(1): 66-76, 2015.
26 Pallagi-Kunstár É. et al., Utility of serum TNF-a, infliximab trough level, and antibody titers in inflammatory bowel disease. World J Gastroenterol. 20(17): (5031-5035), 2014. **27 Khanna R., et al., Therapeutic Drug Monitoring of TNF Antagonists in Inflammatory Bowel Disease. Gastroenterology & Hepatology, August (478-489),2014. **
28 Krajcovicova A. et al., Delayed hypersensitivity reaction after initial dose of infliximab: a case report. European Journal of Gastroenterology& Hepatology 26:(485-487), 2014.
29 Erdemli Ö., et al, In vitro evaluation of effects of sustained anti-TNF release from MPEG-PCL-MPEG and PCL microspheres on human rheumatoid arthritis synoviocytes.2014. Journal of Biomaterials Application, 29(4):524-42, 2014. **
30 Avdeeva A.A., Aleksandrova E.N., Karateev D.E., Luchikhina E.L., Novikov A.A., Cherkasova M.V., Nasonov E.L. EFFICACY OF ADALIMUMAB IN EARLY RHEUMATOID ARTHRITIS IN RELATION TO ITS SERUM LEVEL AND THE PRESENCE OF ANTI-DRUG ANTIBODY. Rheumatology Science and Practice. 52(6):624–630. 2014 Russian, English abstract
31 Bortlik M, et al, Impact of Anti-Tumor Necrosis Factor Alpha Antibodies Administered to Pregnant Women With Inflammatory Bowel Disease on Long-term Outcome of Exposed Children. Inflamm Bowel Dis 20 : (495-451), 2014.
32 Jung Y et al., “Temperature-modulated noncovalent interaction controllable complex for the long-term delivery of etanercept to treat rheumatoid arthritis”, J. Control. Release 2013.
33 Gutierrez A, et al, Genetic susceptibility to increased bacterial translocation influences the response to biological therapy in patients with Crohn’s disease, Gut 0:1–9, 2013. **
34 Grosen A., et al, Infliximab concentrations in the milk of nursing mothers with inflammatory bowel disease, J Crohns Colitis 2013. **
35 Romero G., et al, Poly(Lactide-co-Glycolide) Nanoparticles, Layer by Layer Engineered for the Sustainable Delivery of AntiTNF-α. Macromol. Biosci. 13: (903–912), 2013.
36 Cheong C, et al, Etanercept Attenuates Traumatic Brain Injury in Rats by Reducing Brain TNF-α Contents and by Stimulating Newly Formed Neurogenesis, Mediators of Inflammation, 2013; Volume 2013, Article ID 620837, 9 pages **
37 Bortlik M et al, “Pregnancy and newborn outcome of mothers with inflammatory bowel diseases exposed to anti-TNF-a therapy during pregnancy: three-center study”, Scandinavian Journal of Gastroenterology. 48: 951–958, 2013
38 Bortlik M, et al, Infliximab trough levelsmay predict sustained response to infliximab in patients with Crohn’s disease, Journal of Crohn’s and Colitis 2012. **
39 Malickova K, et al, Phosphatidylserine-dependent anti-prothrombin antibodies (aPS/PT) in infliximab-treated patients with inflammatory bowel diseases, Autoimmun Highlights, 2012. **
40 Takahashi H, et al, Plasma trough levels of adalimumab and infliximab in terms of clinical efficacy during the treatment of psoriasis, Journal of Dermatology 2012; 39: 1- 4. **
41 Seok Lee Y, et al, “Efficacy of Early Infliximab Treatment for Pediatric Crohn’s Disease: A Three-year Follow-up”, Pediatric Gastroenterology, Hepatology & Nutrition 2012; 15(4):243-249 **42 Molnar T, et al, “Importance of trough levels and antibody titers on the efficacy and safety of Infliximab therapy in inflammatory bowel disease”, Z Gastroenterol 2012; 50 – A5343 Kato S, et al, “Elevated Serum IgE Prior to Acute Severe Infusion Reaction During Infliximab Maintenance Therapy in a Crohn’s Disease Patient”, Crohn’s & Colitis Foundation of America 201144 Adisen E, et al, “Anti-infliximab antibody status and its relation to clinical response in psoriatic patients”: A pilot study, Journal of Dermatology 2010; 37: 708–713.
Poster:
1 Malickova K, et al,. Monitoring patients treated with infliximab: Assessing Anti-Infliximab antibodies, Czech Republic. EUROMEDLAB. Autoimmune diseases 2009. S1152 Malickova K, et al, Formation of antiphospholipid antibodies and antibodies to infliximab in anti-TNF-alpha antibody-treated patients with inflammatory bowel diseases, Charles University in Prague Czech Republic (2011).
2 Malickova K, et al, Relationship between serum trough infliximab levels, serum antibodies to infliximab, serum albumin levels and clinical response to infliximab treatment in patients with inflammatory bowel diseases, Charles University in Prague Czech Republic. (2011)
3 Lukas M, et al, Anti-infliximab antibodies in routine clinical practice is it worth to assess them, Czech Republic
4 Duricova D, et al, Predictors of sustained response to infliximab with Crohn’s disease: A single cohort study, Czech Republic
5 Bodini G, et al, Correlation between Adalimumab trough serum concentration, Anti-Adalimumab antibodies and TNF-Alpha levels with clinical outcome in patients affected by Crohn’s disease,. United European Gastroenterology. Italy 2013.
6 Julsgaard M, et al, Time since last drug exposure in pregnancy determines Adalimumab and Infliximab levels in neonates(Era Study), Italy 2014
7 Szepes Z., et al, Clinical utility of measuring serum TNF alpha level, anti TNF alpha levels and antibody titers in critical situations in inflammatory bowel disease and in psoriasis, ECCO 2014 Inflammatory Bowel Disease.
8 Julsgaard M., et al, Intra-uterine Exposure to Anti-TNF-alpha therapy(ERA study):Infliximab and adalimumab cord blood levels correlate with maternal levels at birth, ECCO 2014 Inflammatory Bowel Disease.
9 Goldberg R., et al, Predictors of sub-therapeutic infliximab oradalimumab trough levels and anti-drug antibodies and their influence on therapeutic decisions, ECCO 2014 Inflammatory Bowel Disease.
10 H. Akbulut, et al: The role of immune system on the efficacy of bevacizumab in patients with metastatic colorectal cancer (mCRC). Medical Oncology, Ankara University School of Medicine, Ankara, Turkey. 1553 P, Annals of Oncology, Volume 27, 2016, 7–11 October 2016, Copenhagen, Denmark.















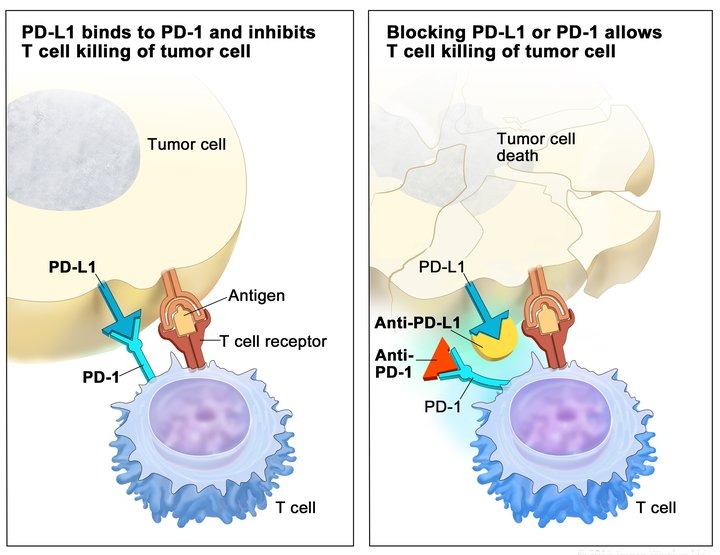






















































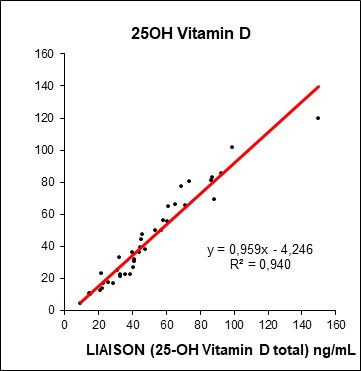














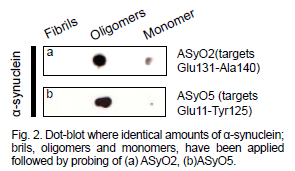















 Utilizza il motore di ricerca dedicato che permette, attraverso filtri specifici, di trovare con facilità il prodotto di interesse e indica quando un anticorpo è altamente validato o è stato recentemente citato in una pubblicazione.
Utilizza il motore di ricerca dedicato che permette, attraverso filtri specifici, di trovare con facilità il prodotto di interesse e indica quando un anticorpo è altamente validato o è stato recentemente citato in una pubblicazione.



 17
17